You are here
FDA head says agency could consider authorization for Covid-19 vaccine before Phase 3 trials are complete, Financial Times reports
Primary tabs
Mon, 2020-08-31 11:59 — mike kraft
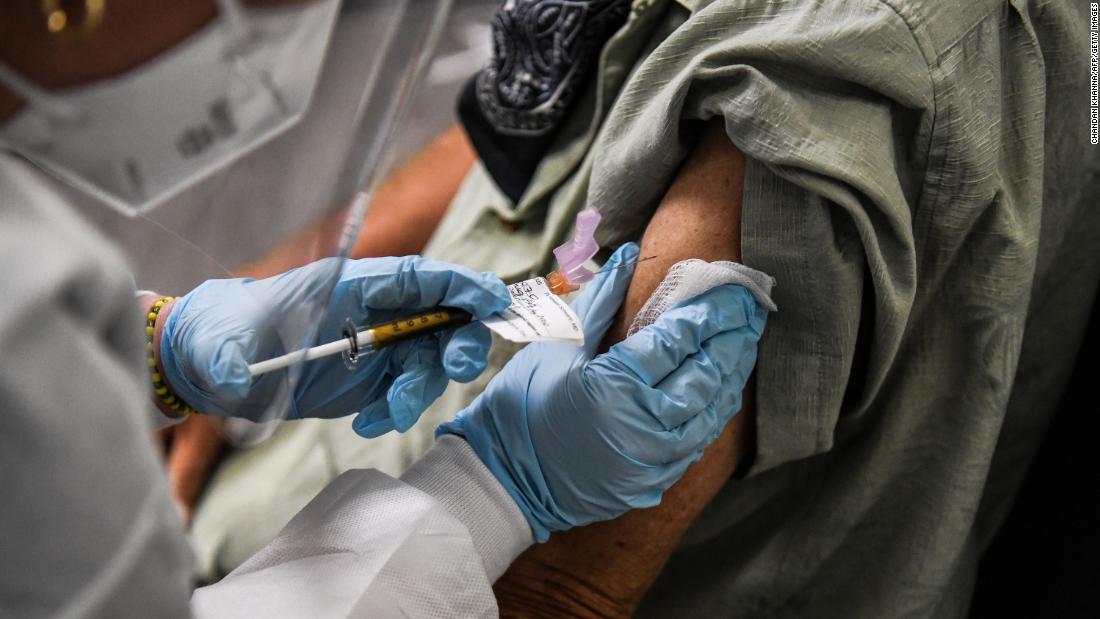 FDA leader says agency could consider authorization for Covid-19 vaccine before Phase 3 trials are complete, Financial Times reports FDA Commissioner, Dr. Stephen Hahn, told the Financial Times that the organization would consider emergency use authorization for a Covid-19 vaccine before Phase 3 trials were completed CNN
FDA leader says agency could consider authorization for Covid-19 vaccine before Phase 3 trials are complete, Financial Times reports FDA Commissioner, Dr. Stephen Hahn, told the Financial Times that the organization would consider emergency use authorization for a Covid-19 vaccine before Phase 3 trials were completed CNN
 FDA leader says agency could consider authorization for Covid-19 vaccine before Phase 3 trials are complete, Financial Times reports FDA Commissioner, Dr. Stephen Hahn, told the Financial Times that the organization would consider emergency use authorization for a Covid-19 vaccine before Phase 3 trials were completed CNN
FDA leader says agency could consider authorization for Covid-19 vaccine before Phase 3 trials are complete, Financial Times reports FDA Commissioner, Dr. Stephen Hahn, told the Financial Times that the organization would consider emergency use authorization for a Covid-19 vaccine before Phase 3 trials were completed CNN CNN)In an interview with the Financial Times, US Food and Drug Administration Commissioner Dr. Stephen Hahn said the agency could consider emergency use authorization or approval for a Covid-19 vaccine before Phase 3 trials are complete.
"It is up to the sponsor [vaccine developer] to apply for authorisation or approval, and we make an adjudication of their application," Hahn told the Financial Times. "If they do that before the end of Phase Three, we may find that appropriate. We may find that inappropriate, we will make a determination."
Hahn noted that an EUA is not the same as FDA approval.
Our emergency use authorisation is not the same as a full approval," he said. "The legal, medical and scientific standard for that is that the benefit outweighs the risk in a public health emergency."
Hahn said the vaccine decision would be based on data, not politics.
"We have a convergence of the Covid-19 pandemic with the political season, and we're just going to have to get through that and stick to our core principles," he told the Financial Times. "This is going to be a science, medicine, data decision. This is not going to be a political decision." ...
Country / Region Tags:
Problem, Solution, SitRep, or ?:
Groups this Group Post belongs to:
- Private group -



Recent Comments